Prof. Dr. Wolfgang Streit
The Streit lab's main aspects of research
If you are interested, here are some media reports showing excerpts from our research:
- DAS! (NDR 06.09.2024) „Plastic-eating bacteria: Enzyme to decompose PET“
- Hamburg Journal (NDR 16.10.2023) „Researchers discover enzyme to break down plastic bottles“ Video
- Tagesschau (NDR 07.10.2023) "Research on the degradation of microplastics"
- Schleswig-Holstein Magazin (NDR 29.09.2023) "Plastic-degrading bacteria in the deep sea" Video
- Leschs Kosmos (ZDF 16.07.2019) "The plastic time bomb: ways out of the crisis"
- Biocatalysts for biotechnology, presented by Prof. Dr. Wolfgang Streit (2019) Video
Further:
Take a one-click look at the PAZy database, which exclusively lists biochemically characterized plastic-active enzymes - curated in collaboration between the University of Stuttgart, Institute of Biochemistry and Technical Biochemistry and the University of Hamburg, Department of Microbiology & Biotechnology.
I. Metagenomics, biotechnology, enzymatic plastic degradation
In one of the central research areas, we use metagenomics (genomes of all microorganisms) to provide new biocatalysts and other valuable biomolecules for various biotechnology applications. A central aspect of our research is the utilization of non-cultivated organisms or their metagenomes for biotechnology. Today we know that 99 % of all microorganisms are not or only with difficulty cultivable. We want to exploit this almost unlimited potential of new enzymes and molecules. In order to use this immense potential, we are developing new technologies on the one hand and identifying many biocatalysts on the other. This is done against the background of both basic and applied research.
- Biocatalysts from the metagenome for the chemical industry: With regard to the identification and supply of biocatalysts, we have worked on relatively different classes of enzymes, but our focus is on the isolation of lipases, esterases and other hydrolases for industrial applications. In this respect we now have one of the largest lipase/esterase enzyme collections with over 150 active and characterize biocatalysts (Metacat collection) and about 80 active cellulolytic enzymes. In addition, there are lactonases, hydrogenases, glycosyltransferases, decarboxlyases and other enzymes, which we have often characterized up to the 3D structure. In our work we are also interested in identifying completely new reactions and enzymes (dark and grey matter proteins). In addition, we usually develop reporter assays for the detection of the reactions in high-throughput procedures. We are constantly expanding our metagenome enzyme collection, which forms the basis for many of our research projects.
- Enzymes for the degradation of synthetic polymers: In the course of the above mentioned metagenome searches we started to isolate PET-degrading enzymes a few years ago. We currently have about 60-PET-active enzymes from a wide variety of organisms that have been identified and partially characterized in our laboratories. These enzymes are currently the focus of two BMBF projects (LipoBiocat and Plastisea) and represent the largest collection of such enzymes worldwide. We are currently extending this work to other plastics for which there are no known enzymes. In principle, we assume that not only the degradation is of interest, but also the use of the enzymes for the synthesis of new biodegradable polymers and materials. This work currently forms the basis for a number of ongoing doctoral theses.
- Establishment of a digital platform for enzyme searches: A challenge in many of our projects, which we often carry out in cooperation with industrial partners, is caused by the relatively long runtimes. The usual development time is usually 3-5 years, calculated from the identification of the enzyme to the first upscaling trials. In order to shorten this period, we are working at full speed on an automated platform. We want to achieve the provision of robust enzymes or entire metabolic pathways for biotechnology in relatively short periods of time of maximum 3-6 months. Here, we are working on the development of in vitro systems in order to gain really fast access to non-cultivated organisms. In combination with sequence-based metgenome searches and in vitro approaches, the aim is to provide a few thousand active enzymes for initial screening in a very short time and only in a second step to enter upscaling. The platform will be available in a few years. Currently we can provide about 30-50 enzymes within a very short time.
II. Biofilms: Analytics, architecture and omics
Microbial biofilms are often unwanted growths on surfaces and they pose a real challenge in both clinical and industrial environments.
- Biofilms - detection and analysis: Biofilm analysis and reliable diagnostics are often very difficult and costly in industry and also in the clinical environment. For this purpose we analyse metagenomes of complex biofilms and thus gain deep insights into the functioning of these consortia. We use the whole range of omics analysis and image-representing methods, such as electron microscopy, are an integral part of such analyses. We have also established EPS analysis.
- Control and management of growth at interfaces: To this end, we are working intensively on the elucidation of processes that play a role in bacterial communication during biofilm formation. In this field of research we are particularly interested in how microorganisms talk to each other. On the one hand, we are looking for new signalling molecules, on the other hand we are also investigating how growth in a biofilm can be specifically controlled. We are working with mixed consortia on the level of entire populations as well as in the field of single cell analysis. In this work we also reconstruct multi-species biofilms and try to simulate the natural conditions in the process or clinical environment. In this way we gain very deep insight into 3D biofilm structures and omics.
- Avoidance of microbial biofilms: In principle, it is better to inhibit biofilms very early in growth. Here we are working together with colleagues from the Chemistry department on both enzymatic and compound-driven approaches to inhibit microbial biofilms. We have achieved initial successes with enzymes that intervene in cell-cell communication, but also with molecules from the group of flavonoids and diorcinols. The long-term goal of this work is to change the biofilm population in such a way that no (hardly) biofilms are formed or that a rapid detachment takes place.
III. Microbial Diversity and 'One Health'
The increasing release of antibiotics and other pollutants has a direct and indirect impact on microbial diversity and promotes the spread of bacterial resistance mechanisms, which pose a high risk to human and animal health. In addition, climate change and other human factors accelerate these effects. There are no clear methods and models to assess the impact of these factors on biodiversity and human health in urban areas.
Therefore, the MOMOBIO2.0 project aims to develop detailed, mainly omics-based datasets and models to track, monitor and predict the urban microbiota and changes in biodiversity and resistance profile in the urban water cycle. The main goal is to use changes in microbial biodiversity as an indirect measure of human and animal health (One Health concept). We establish genetic markers to track changes in biodiversity and resistance within wastewater streams and surface waters.
This network project is being carried out in close cooperation with all relevant partners in relation to wastewater and water flows in Hamburg, including the Universitätsklinikum Hamburg-Eppendorf (UKE), Hamburg Wasser (HW), research teams from the University of Hamburg (UHH), the local authorities (HU), the NGO Life Science Nord (LSN) and data from the Hamburg City Health Study (HCHS).
The data obtained in the project and the newly developed models should make it easier for decision-makers in politics and industry to take appropriate measures to maintain water quality and thus contribute to the health of humans and animals. The project partner UKE contributes to the project with its expertise in high-throughput cultural methods (culturomics), quantitative PCR and bioinformatic analysis of complex genome and metagenome data.
Available range of methods
Methodologically, the lab is very broadly based due to the diversity of topics and has a great deal of expertise in the fields of DNA and RNA technologies, genomics and metagenomics, transcriptomics, GC and HPLC analysis and protein purification. In addition, the laboratory has expertise in the handling of a number of model organisms that are particularly important in biotechnology and industrial microbiology. These include Pseudomonas sp., Burkholderia sp. and others. Within the scope of biotechnological work, we also develop microorganisms for production. We have established genetic systems for all organisms. Finally, in recent years we have just begun to establish single cell technology in the laboratory and have established the first laser trapping system for this purpose. We also have 3D biofilm systems and a confocal laser scanning system for biofilm analysis. Furthermore, we often develop new techniques for the detection of enzyme activities in metagenome banks.
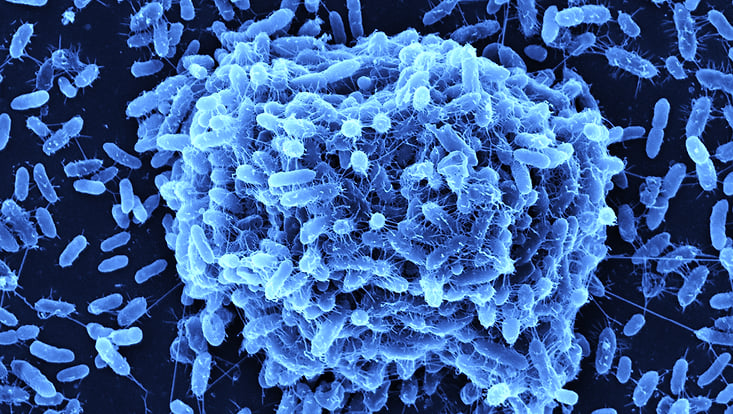
Photo: UHH/Mikrobiologie
Bacterial pathogens can adhere and form biofilms on host tissues such as the respiratory tract, skin, as well as on abiotic surfaces such as medical devices and implants. Immunocompromised patients suffer a high risk of hospital acquired infections as result of these biofilms. Our main research focus lies in establishing multispecies biofilm models with the following model organisms Stenotrophomonas maltophilia, Pseudomonas aeruginosa , Staphylococcus aureus and the fungi Candida albicans. We use these biofilm models to investigate interspecies interaction, layer formation , gene expression profiles using RNA seq analysis and the effect of strain specific bacteriophages on biofilm formation and structures.
The picture shows a biofilm formation of the human pathogen Stenotrophomonas maltophilia.
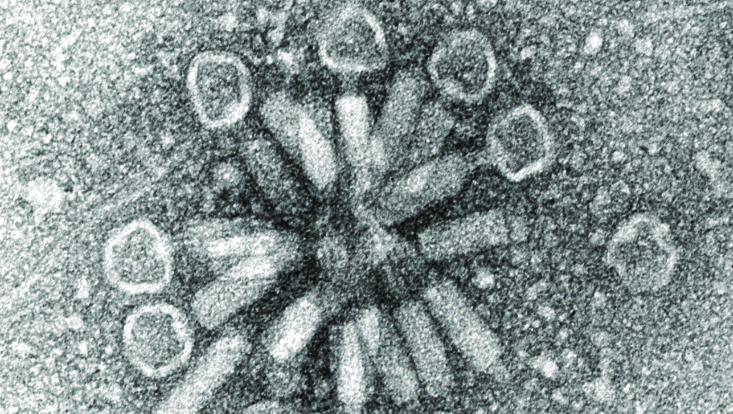
Photo: UHH/Mikrobiologie
Stenotrophomonas maltophilia phage
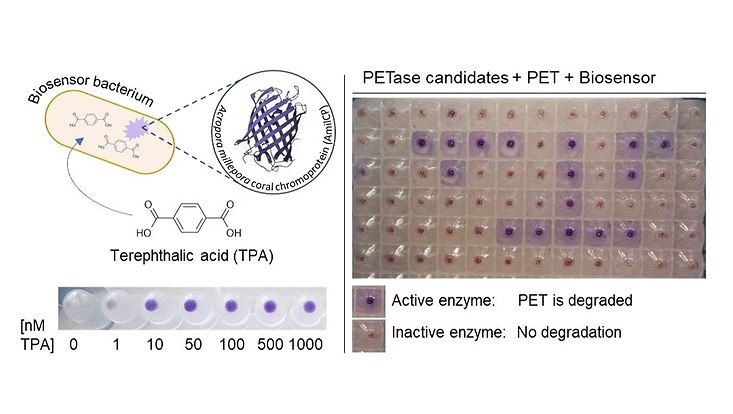
Photo: UHH/Mikrobiologie
Biosensors for the discovery of novel PET-degrading enzymes. A Bacterial Biosensor produces a visible blue coral chromoprotein when it senses the PET degradation product Terephthalic acid (TPA). Sensitive screening systems like these can help to discover new PETases with high and low activities from many different sources. (Applied and Environmental Microbiology, Vol. 89, No. 1, Dierkes et al., 2022)
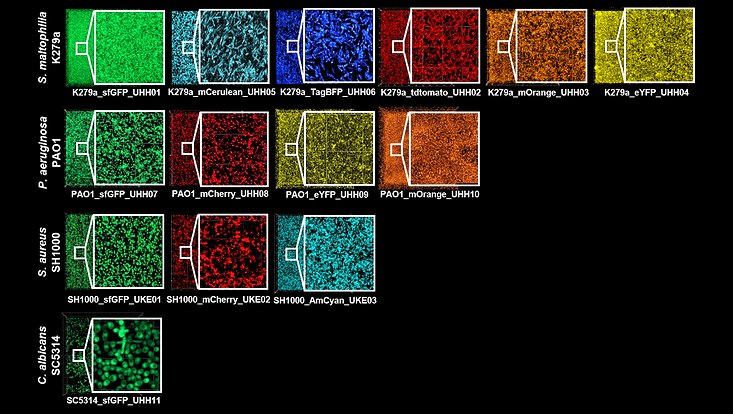
Photo: UHH/Mikrobiologie
Microscopic images of fluorescently labeled pathogens in biofilm. The different species Stenotrophomonas maltophilia, Pseudomonas aeruginosa and Candida albicans were identified by integrating the fluorescence gene into the respective genome fluorescently labeled. In Staphylococcus aureus, on the other hand, integration of the fluorescent gene occurred extrachromosomally via plasmid PCM29. (Genetics and Molecular Biology, Alio et al., 2023)

Photo: UHH/Mikrobiologie
Microscopic images of a triple species biofilm after 24h, 48h and 72h. The biofilm consists of the pathogens Stenotrophomonas maltophilia K279a tdTomato (red), Staphylococcus aureus SH1000 AmCyan (cyan) and Candida albicans SC5314 sfGFP (green).
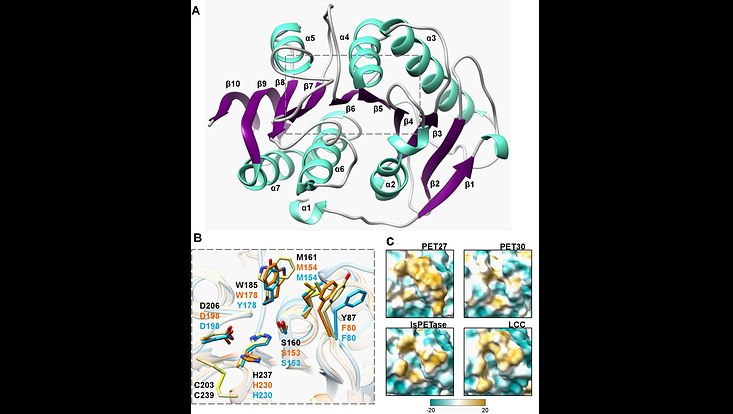
Photo: UHH/Mikrobiologie
Crystal structure of the PET-hydrolyzing bacteroidetal PET30∆PorC including active site and hydrophobicity comparison. (A) Overall structure of PET30∆PorC. The structure was solved by X-ray crystallography to a resolution of 2.1 Å. (B) Comparison of active site residues. All three enzymes PET30∆PorC (light blue), PET27 (orange) and IsPETase (light yellow) have the typical residues of Ser-hydrolases at the catalytically active positions (Ser, His and Asp). The residues of IsPETase are indicated in black. (C) Surface hydrophobicity around the tunnel leading to the active site of four PET-degrading enzymes. Hydrophilic regions are displayed in turquoise and hydrophobic in gold. Taken from Zhang et al. 2022 (Front. Microbiol. 12:803896. doi: 10.3389/fmicb.2021.803896)

Photo: Pablo Pérez-García
Plastic polluted beach in Mallorca, July 2021
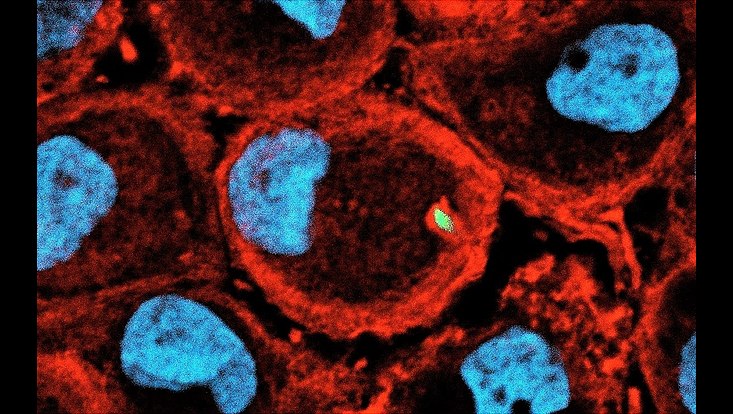
Photo: UHH/Mikrobiologie
Stenotrophomonas maltophilia is a gloabl emerging opportunistic pathogen that is of clinical relevance. Macrophages pose the first line of immune defence during bacterial infection. Employing various clinical isolates of S. maltophilia we are currently investigating the interaction of this bacteria with primary human macrophages. Staining Macrophage F-Aktin with Phalloidin 568 (red) cell nucleus with DAPI (blue), S. maltophilia are tagged with GFP (green)
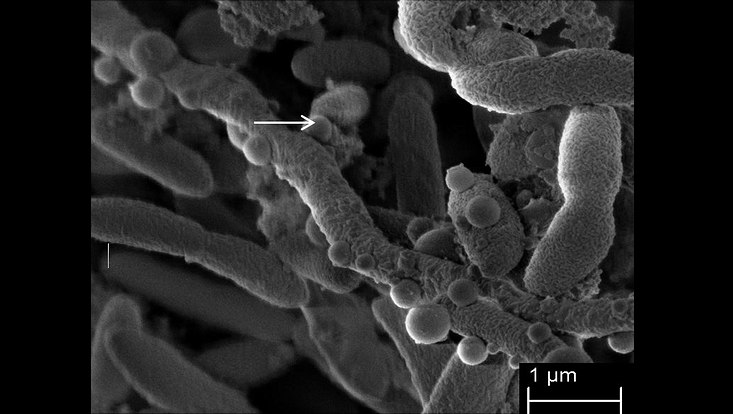
Photo: UHH/Mikrobiologie
Stenotrophomonas maltophilia forming very long filamentous cells and outer membrane vesicles. Image derived from Abda et al., 2015
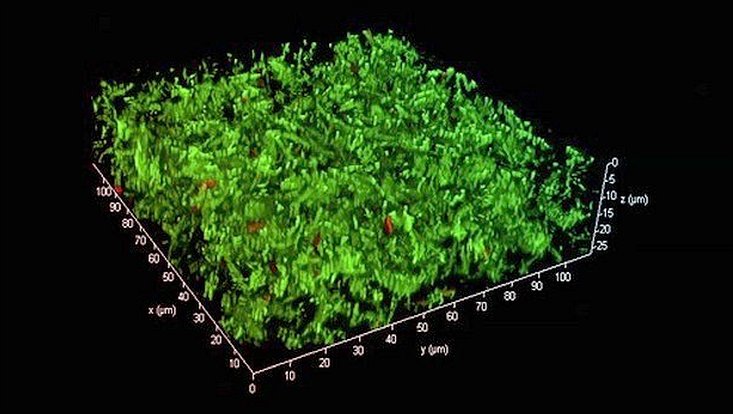
Photo: UHH/Mikrobiologie
3D-structure of a bacterial biofilm in a flow cell produced by Stenotrophomonas maltophilia K279a, an emerging pathogen (Cells were visualized with the life dead stain). The biofilms of Stenotrophomonas maltophilia clinical isolates were grown in flow cells under flow conditions at 28 °C for 72 hours. (Applied and Environmental Microbiology Vol. 86, No. 24, Alio et al., 2020)
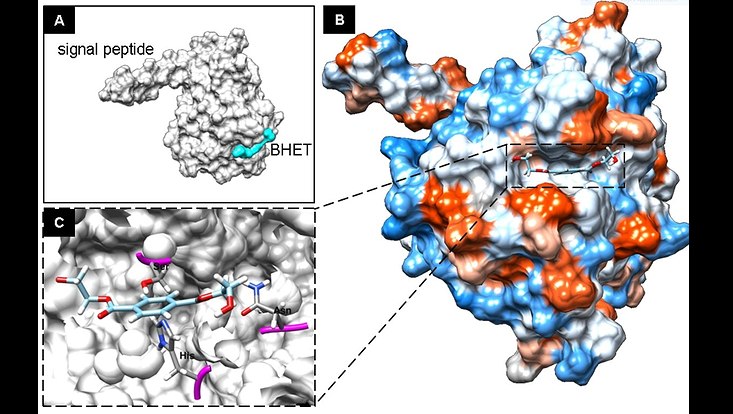
Photo: UHH/Mikrobiologie
3D-Structure prediction model of PET2 a metagenome-derived PET esterase and Bishydroxyethyl Therepthalic (BHET) acid fitting into the active site. The image was derived from Danso et al., 2018

Photo: UHH/Mikrobiologie
Cellulolytic bacteria forming colonizing cellulose fibres (unpublished image)

Photo: UHH/Mikrobiologie
Comamonas sp. forming biofilms on PET fibres. From the cover of AEM 19 and modified after Danso et al., 2019
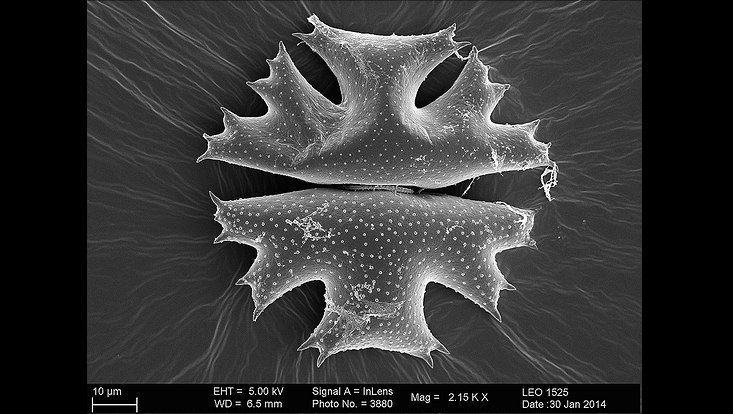
Photo: UHH/Mikrobiologie
Microbes colonizing microalga surfaces of Micrasterias crux melitensis. Small image shows a magnification of the core region of the microalga (unpublished).
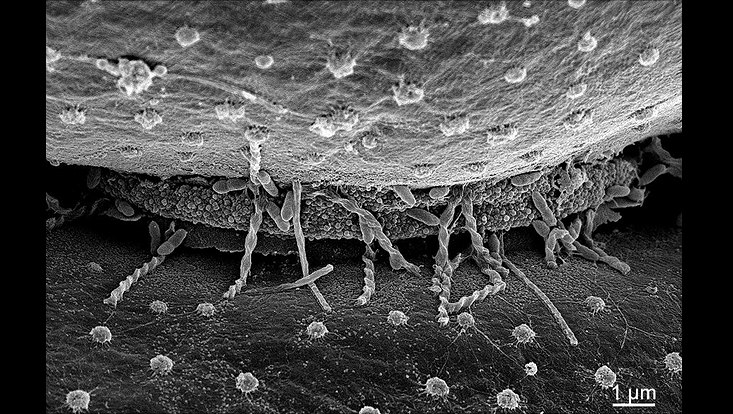
Photo: UHH/Mikrobiologie
A zoom into the center of the microalga Crux melitensis

Photo: UHH/Mikrobiologie
Mining metagenomes for active hydrolases. Image derived from the cover of Green Chemistry issue 7, 2009 and Pottkämper et al., 2009
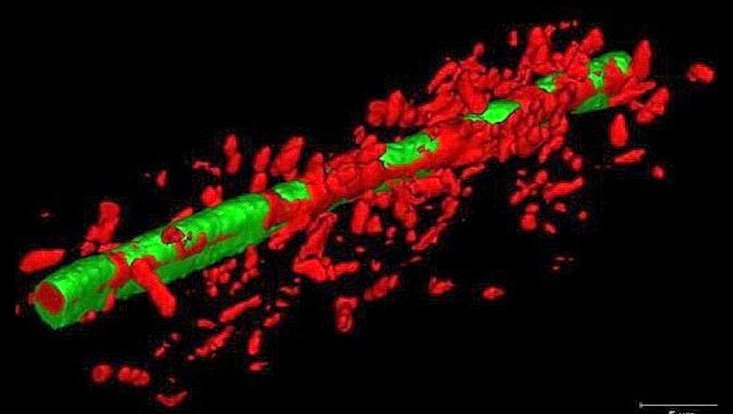
Photo: UHH/Mikrobiologie
Mikrobieller Biofilm von Janthinobacterium sp HH102 (rot) auf Filamenten des pflanzenpathogenen Pilzes Fusarium graminearum (grün) (Haack et al., 2016)
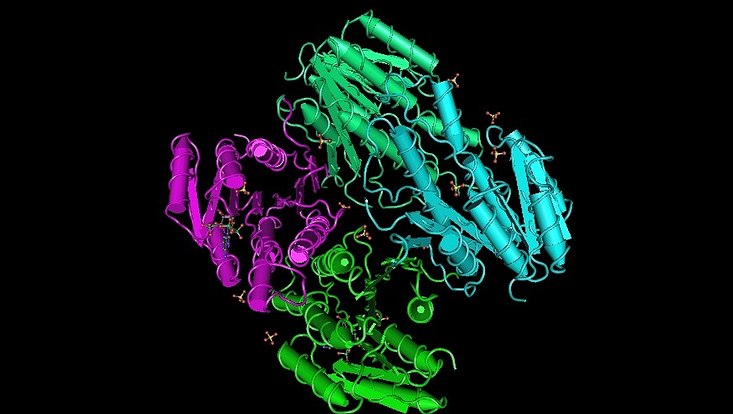
Photo: UHH/Mikrobiologie
3D-structure of a metagenome-derived oxidoreductase 3RKR involved in quenching auf Quorum sensing molecules in gram negative bacteria (Bijtenhoorn et al., 2011)
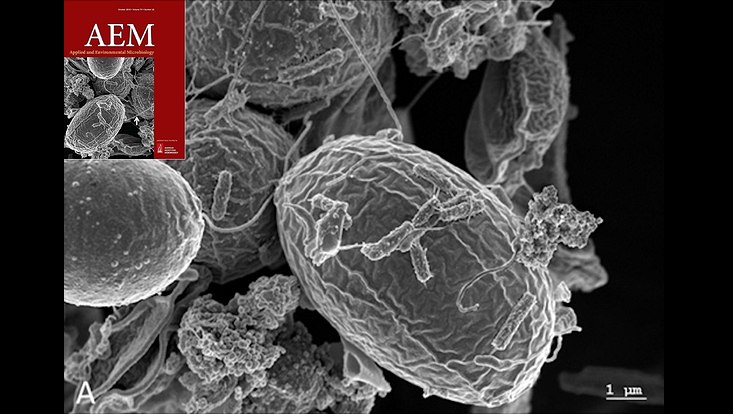
Photo: UHH/Mikrobiologie
Microbes colonizing microalga surfaces. The image was derived from Krohn et al.., 2013 and the cover image of AEM issue 79, (20) 2013.
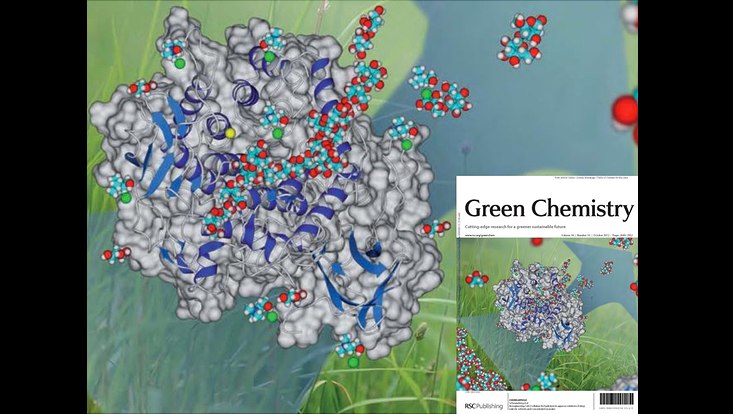
Photo: UHH/Mikrobiologie
3D-structure of the metagenome-derived and optimized cellulase CelA2 from the cover of Green Chemistry issue 10, 2012 and Lehmann et al., 2012
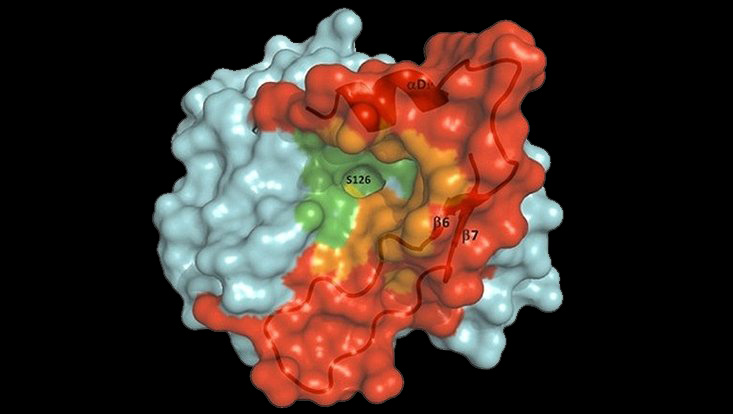
Photo: UHH/Mikrobiologie
3D-structure of the metagenome derived Lipase LipS (Chow et al., 2012, PDB: 4FBL). LipS is a true lipase derived from a non-cultivated Symbiobacterium sp. It has a high potential for application in biotechnology.
