Research
The major goal of our research is to understand the molecular structure, function and regulation of redox systems at the plant cell membrane that are involved in nutrient uptake, ROS production, signaling and redox homeostasis.
The standard-system - structure & function
 |
|
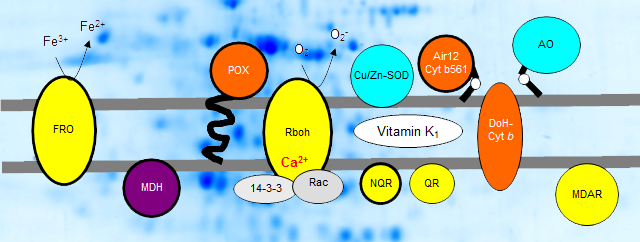
The plasma membrane builds the outer permeability barrier of the cell that achieves important functions in cell signaling, transport processes, plant development and stress response. About 40 years ago, a constitutive transmembrane electron transport system - the so-called standard system - was demonstrated for plasma membranes of eucaryotic cells. Besides the standard system, a second transmembrane electron transport system was demonstrated in root plasma membranes of non-grass monocots and dicots that is induced by iron deficiency (so-called turbo system or ferric-chelate reductase, FRO). Later on respirator burst oxidase homologs (Rboh) have been identified in plants by molecular biological approaches. Meanwhile several redox proteins could be identified in highly enriched plasma membrane fractions prepared by aqueous polymer two-phase partitioning (APTPP): b-type cytochromes and NAD(P)H oxidoreductases. The structure, electron transfer mechanism and function of plasma membrane-bound redox systems are one of our major topics. |
References
S. Lüthje, P. Van Gestelen, M.C. Córdoba-Pedregosa, J.A. González-Reyes, H. Asard, J.M. Villalba, M. Böttger (1998) Quinones in plant plasma membranes – a missing link? Protoplasma 205:43–51
M. Menckhoff, S. Lüthje (2004) Transmembrane electron transport in sealed and NAD(P)H-loaded right-side-out plasma membrane vesicles isolated from maize (Zea mays L.) roots. Journal of Experimental Botany 55:1343–1349
Lj. Menckhoff, N. Mielke-Mielke-Ehret, F. Buck, M. Vuletic, S. Lüthje (2013) Plasma membrane-associated malate dehydrogenase of maize (Zea mays L.) roots: Native versus recombinant protein. Journal of Proteomics 80C:66-77.
S. Lüthje, B. Möller, F.C. Perrineau, K. Wöltje (2013) Redox pathways in plant plasma membranes and oxidative stress. Antioxidants and Redox Signaling 18:2163-83.
S. Lüthje, D. Hopff, A.K. Schmitt, C.-N. Meisrimler, Lj. Menckhoff (2009) Hunting for low abundant redox proteins in plant plasma membranes. Journal of Proteomics 72: 475-483
A. Hofmann, S. Wienkoop, S. Lüthje (2022) Hypoxia-induced aquaporins and regulation of redox homeostasis by a trans-plasma membrane electron transport system in maize roots. Antioxidants 11 (5), 836
Iron homeostasis
  |
|
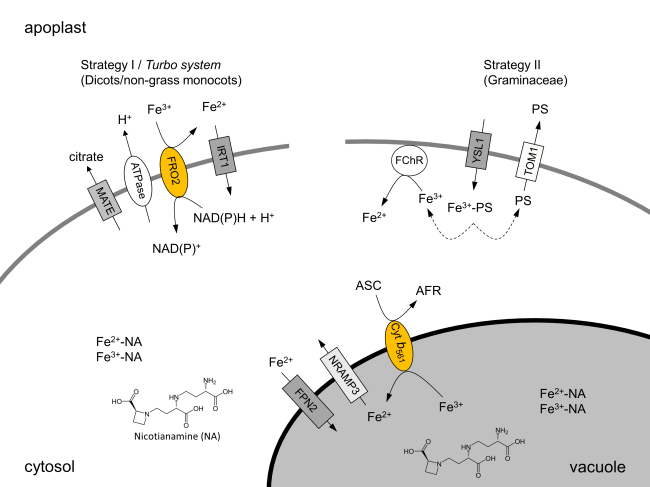
Studies of our team showed alterations in the plasma membrane proteome of iron uptake strategy I (pea, Pisum sativum) and strategy II plants (maize, Zea mays) by iron deficiency or iron toxicity for the first time. Peroxidases, redox systems and several other proteins are regulated under those conditions. The combination of iron deficiency with elicitor treatment support important functions of heme containing enzymes, but also of other plasma membrane proteins, in pathogen response. C.N. Meisrimler, S. Planchon, J. Renaut, K. Sergeant, S. Lüthje (2011) Alteration of plasma membrane-bound redox systems of iron deficient pea roots by chitosan. Journal of Proteomics, 74: 1437-1449 D. Hopff, S. Wienkoop, S. Lüthje (2013) The plasma membrane proteome of maize roots grown under low and high iron conditions. Journal of Proteomics 91:605-18. C.N. Meisrimler, S. Wienkoop, D. Lyon, C.M. Geilfus, S. Lüthje (2016) Long-term iron deficiency: Tracing changes in the proteome of different pea (Pisum sativum L.) cultivars. Journal of Proteomics 140:13-23. E. Gutierrez-Carbonell, D. Takahashi, S. Lüthje, J.A. Gonzalez-Reyes, S. Mongrand, B. Contreras-Moreira, A. Abadía, M. Uemura, J. Abadía, A.F. López-Millán (2016) A shotgun proteomic approach reveals that Fe deficiency causes marked changes in the protein profiles of plasma membrane and detergent resistant microdomain preparations from Beta vulgaris roots. Journal of Proteome Research, 15(8):2510-24. doi: 10.1021/acs.jproteome.6b00026. |
Class III peroxidases - ROS production & scavenging
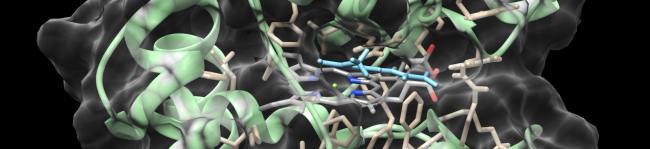
Our team was the first that identified membrane bound heme peroxidases in plant plasma membranes. Class III peroxidases are a multigene family with at least 143 isoenzymes in maize. It appears that these enzymes build a cellular network of ROS scavenging and producing enzymes (PeroxiNET) that has to be tightly regulated.
We established protocols for cell fractionation and gel-based proteomics to investigate the regulation and function of peroxidases within this network in plant development and oxidative stress. Beside studies on the structure and localization of these proteins by in silico analysis and GFP-fusions, heterologous expression and gene silencing (RNAi) are used for functional analysis.
- S Lüthje, K Ramanathan (2020) In Silico Analysis of Class III Peroxidases: Hypothetical Structure, Ligand Binding Sites, Posttranslational Modifications, and Interaction with Substrates. Plant Proteomics, 325-339
- A Hofmann, S Wienkoop, S Harder, F Bartlog, S Lüthje (2020) Hypoxia-Responsive Class III Peroxidases in Maize Roots: Soluble and Membrane-Bound Isoenzymes. International Journal of Molecular Sciences 21 (22), 8872
- S Lüthje, T Martinez-Cortes (2018) Membrane-bound class III peroxidases: unexpected enzymes with exciting functions. International Journal of Molecular Sciences 19 (10), 2876
- S Lüthje, CN Meisrimler, D Hopff, T Schütze, J Köppe, K Heino (2013) Class III peroxidases. In: Jorrin-Novo J., Komatsu S., Weckwerth W., Wienkoop S. (eds) Plant Proteomics. Methods in Molecular Biology (Methods and Protocols), vol 1072, pp. 687-706. Humana Press, Totowa, NJ
- A Mika, MJ Boenisch, D Hopff, S Lüthje (2010) Membrane-bound guaiacol peroxidases from maize (Zea mays L.) roots are regulated by methyl jasmonate, salicylic acid, and pathogen elicitors. Journal of Experimental Botany 61 (3), 831-841
- A Mika, F Buck, S Lüthje (2008) Membrane-bound class III peroxidases: identification, biochemical properties and sequence analysis of isoenzymes purified from maize (Zea mays L.) roots.Journal of Proteomics 71 (4), 412-424
- A Mika, S Lüthje (2003) Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiology 132 (3), 1489-1498
Biotic & abiotic stress
 |
|
Plants are sessil organisms that have to cope with multiple abiotic and biotic stress factors during their life cycle. We are interested in iron homeostasis, toxic elements, weather extremes and multiple stresses that occurred during plant growth and development. Emissions of industrial activities cause accumulation of cadmium and other toxic elements in the environment. Ions of these elements are taken up by unspecific transporters. Inside the cell, they cause oxidative stress and thereby changes in the cellular redox state. The molecular mechanisms of these reactions are not very well understood. So far, total peroxidase activity of samples is used as a general stress marker for many stress factors. My team established protocols to study the regulation of different peroxidase isoenzymes side by side in the same sample. We found that abundance and activity of different soluble, cell wall and membrane bound class III peroxidases changed under cadmium exposure. Due to changing climate heat, drought and flooding events increases in northern hemisphere. Additionally, the use of heavy machinery in agriculture cause soil compaction, which supports waterlogging. Flooding provokes alterations in pH and oxygen levels (hypoxia and anoxia) in the soil and thereby iron toxicity. A recent study of our team showed alterations in abundance and activity of soluble class III peroxidasesand plasma membrane bound redox systems in maize by waterlogging. References A. Hofmann, S. Wienkoop, S. Lüthje (2022) Hypoxia-induced aquaporins and regulation of redox homeostasis by a trans-plasma membrane electron transport system in maize roots. Antioxidants 11 (5), 836 C.N. Meisrimler, S. Planchon, J. Renaut, K. Sergeant, S. Lüthje (2011) Alteration of plasma membrane-bound redox systems of iron deficient pea roots by chitosan. Journal of Proteomics, 74: 1437-1449 D. Hopff, S. Wienkoop, S. Lüthje (2013) The plasma membrane proteome of maize roots grown under low and high iron conditions. Journal of Proteomics 91:605-18. C.N. Meisrimler, S. Wienkoop, D. Lyon, C.M. Geilfus, S. Lüthje (2016) Long-term iron deficiency: Tracing changes in the proteome of different pea (Pisum sativum L.) cultivars. Journal of Proteomics 140:13-23. E. Gutierrez-Carbonell, D. Takahashi, S. Lüthje, J.A. Gonzalez-Reyes, S. Mongrand, B. Contreras-Moreira, A. Abadía, M. Uemura, J. Abadía, A.F. López-Millán (2016) A shotgun proteomic approach reveals that Fe deficiency causes marked changes in the protein profiles of plasma membrane and detergent resistant microdomain preparations from Beta vulgaris roots. Journal of Proteome Research, 15(8):2510-24. doi: 10.1021/acs.jproteome.6b00026. C.N. Meisrimler, F. Buck, S. Lüthje (2014) Alterations in Soluble Class III Peroxidases of Maize Shoots by Flooding Stress. Proteomes 2 (3), 303-322 |
